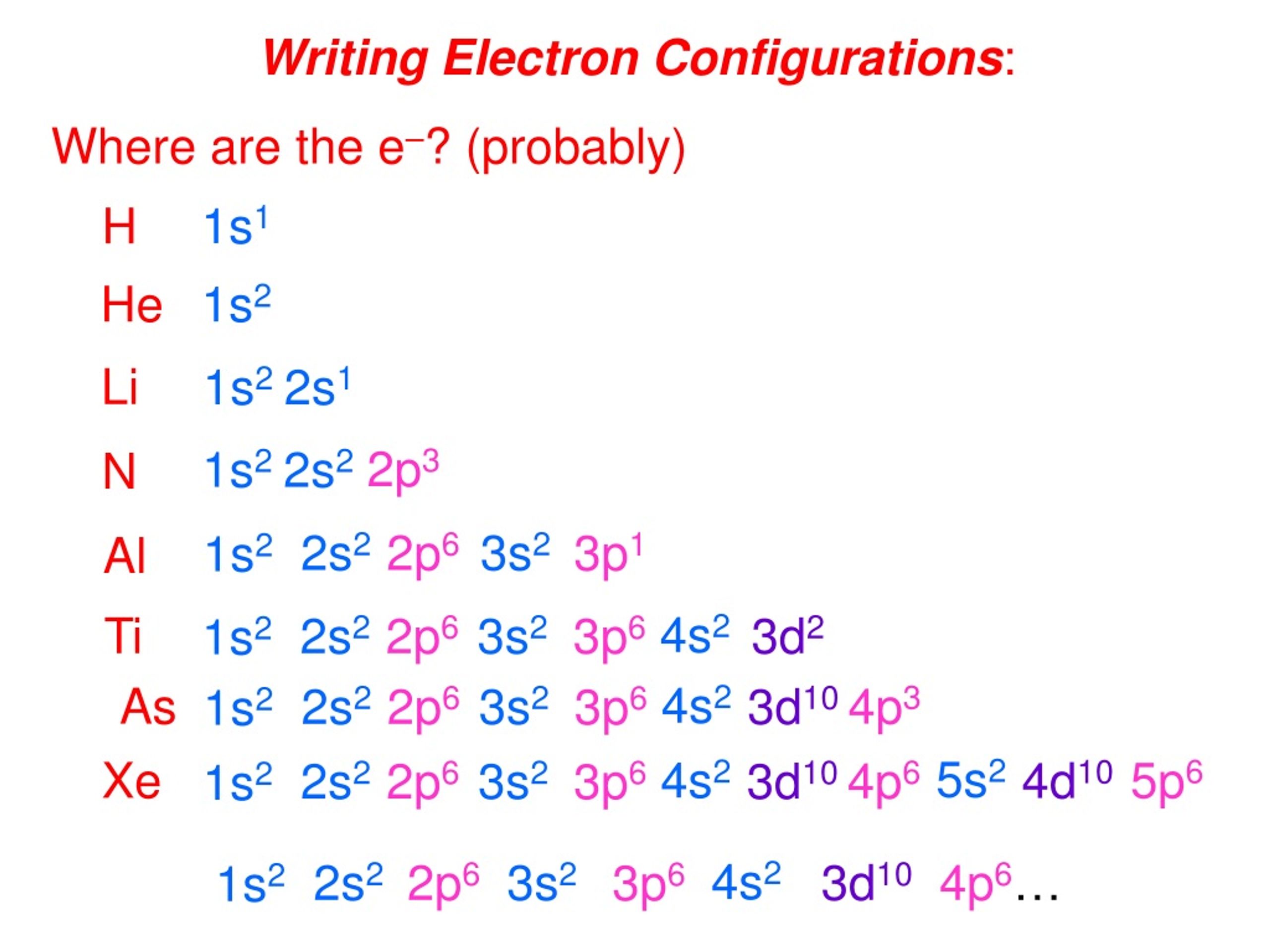
This comprehensive guide will explore the electron configuration of a neutral boron atom, delving into the principles behind it, its implications for chemical bonding, and its relationship to other. 12 jun 2023 the electron configuration of a neutral atom of boron (atomic number 5) is 1s 2s 2p. This indicates that boron has 5 electrons, with 2 in the first shell and 3 in the second shell,. The electron configuration for a neutral atom of boron, which has 5 electrons, is 1s2 2s2 2p1. Since boron has 5 electrons, there is one more electron to distribute.
Putting it all together, the electron configuration of boron is: 5 jul 2024 to write the electron configuration for boron, the first two electrons would enter the 1s orbital. The next two electrons will enter the 2s orbital and the remaining one electron will enter. Writing electron configurations for ions negative ions: To write the electron configuration, add electrons to the last unfilled subshell, starting where the neutral atom left off.